Description
What is subutex
SUBUTEX (buprenorphine) is a sublingual tablet. Tablet is an uncoated, oval white, flat-edged tablet, debossed with a word identifying the product and strength on one side. It contains buprenorphine HCl, a partial agonist at the mu-opioid receptor, and is available in two dosage strengths, 2 mg buprenorphine and 8 mg buprenorphine (as the free base, equivalent to 2.16 mg buprenorphine hydrochloride USP and 8.64 mg buprenorphine hydrochloride USP). Each tablet also contains lactose, mannitol, cornstarch, povidone K30, citric acid, sodium citrate and magnesium stearate.
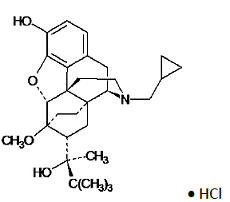
Chemically, buprenorphine HCl is (2S)-2-[17-Cyclopropylmethyl-4,5α-epoxy-3-hydroxy-6-methoxy6α,14-ethano-14α-morphinan-7α-yl]-3,3-dimethylbutan-2-ol hydrochloride.
Indication
SUBUTEX is indicated for the treatment of opioid dependence. Counselling and psychosocial support should be include when SUBUTEX treatment starts.
Drug Addiction Treatment Act
Under the Drug Addiction Treatment Act (DATA) codified at 21 U.S.C. 823(g). Prescription use of this product in the treatment of opioid dependence is limited to healthcare providers who meet certain qualifying requirements. Equally those who have notified the Secretary of Health and Human Services (HHS). This is in their intent to prescribe this product for the treatment of opioid dependence and have a unique identification number included on every prescription.
Important Dosage And Administration Instructions
Administer SUBUTEX sublingually as a single daily dose.
Consider the frequency of visits before the prescription.
Induction
Consider the type of opioid dependence prior to induction. The time since last opioid use, and the degree or level of opioid dependence.
Patients Dependent On Heroin Or Other Short-Acting Opioid Products
Rapidly achieve titrated clinical effective dose is a recommendation. Give 2 mg to 4 mg incremental doses on the initial day of treatment. In some studies, gradual induction over several days led to a high rate of dropout during the induction period.
In a one-month study, patients received 8 mg of SUBUTEX on Day 1 and 16 mg SUBUTEX on Day 2. From Day 3 onward, patients received either SUBOXONE sublingual tablet or SUBUTEX as Day 2 based on their assigned treatment. accomplish Buprenorphine induction over 3-4 days.
Patients Dependent On Methadone Or Other Long-Acting subutex Products
Equally, Patients dependent upon methadone or other long-acting opioid products may be more susceptible to precipitated and prolonged withdrawal. Especially during induction than those on short-acting opioid products. Only administer subutex when moderate opioid withdrawal appear. Generally, not less than 24 hours after the patient last used a long-acting opioid product.
There is little controlled experience with the transfer of methadone-maintained patients to buprenorphine. Available evidence suggests that withdrawal signs and symptoms are possible during induction onto buprenorphine. Withdrawal appears more likely in patients maintained on higher doses of methadone (>30 mg) and when the first buprenorphine dose is administered shortly after the last methadone dose.
Other products you can shop with us



Reviews
There are no reviews yet.